Research
The goal of our lab is to develop vaccines and therapeutics for HIV/AIDS and other infectious diseases
Developing HIV treatment strategy
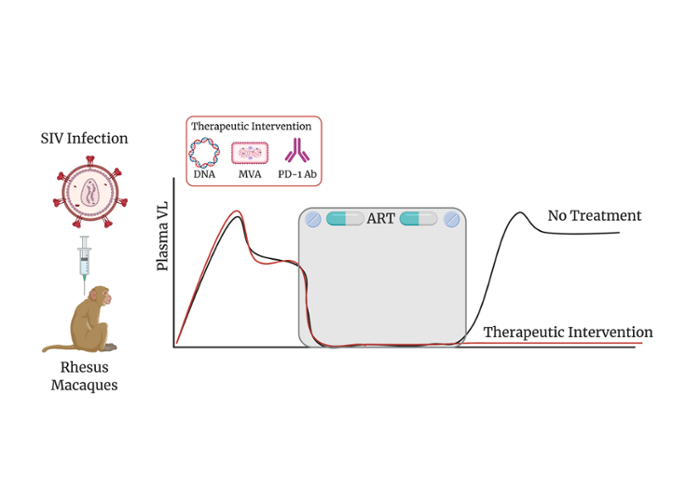
In this arm of our research, our goal is to design and evaluate vaccines and other cure strategies to achieve functional cure of HIV in SIV infected rhesus macaques. Cellular immune responses are critical for the control of HIV replication, particularly, CD8 T cells, play a major role in restricting the virus growth and eliminating virus-infected cells. In the presence of antigen and inflammatory signals, cognate naïve CD8 cells expand and traffic to infection sites where they lyse virus-infected host cells. However, CD8 T cells from HIV-infected individuals have been shown to be non-functional. Our efforts are focused on reprogramming these cells so they can clear the virus-infected cells and help clearing the virus. Chronic HIV/SIV infection is characterized by dysfunctional anti-viral immunity, CD8 T cell exhaustion, persistent viral reservoirs, and failure to induce anti-viral CD8+ T cells in lymphoid tissue, where majority of the reservoirs persist. These anomalies exist even under anti-retroviral therapy (ART), limiting complete HIV cure. Previous studies by our group have shown that CD8 T cell functions can be improved by PD-1 blockade with or without ART alone or in combination with therapeutic vaccination, which in turn increase anti-viral immunity in SIV infected rhesus macaques (RMs) (Velu et al., 2009; Mylvaganam et al., 2018; Rahman et al., 2021). Building on these studies, we are currently exploring new generation therapeutic vaccines, latency reversal agents and PD-1 blockade to achieve functional cure in SIV infected rhesus macaque models.
Understanding B and T Cell Biology for prevention and treatment of HIV infections
As a part of Emory Consortium for Innovative AIDS Research (CIAR) in non-human primates, we are developing prophylactic vaccines for HIV using a monkey model. Our strategy is to induce a balanced and sustained humoral and cellular immunity at the site of virus entry (Arunachalam et al, 2019). We are working on developing/testing immunogens and their delivery through various routes. Our immunogens are based on our DNA/MVA platform for induction of T-cell response and, potent soluble HIV envelope protein trimers for induction of antibody (neutralizing and effector) responses. We aim to test these immunogens in combinations with different adjuvants to achieve higher and durable antibody responses. Our focus is to evaluate the cellular immune responses at single cell level induced post vaccination/challenge at mucosal and lymphoid tissues.
Developing MVA based vaccines against SARS-CoV-2
The newly emerged coronavirus, SARS-CoV-2, a causative agent of the COVID-19 disease impacted health and economy worldwide. As of January 24, 2021, the nCoV-19 has infected nearly 96 million people resulting in 2 million deaths worldwide. My research focuses on designing a combination of vaccination approaches to tackle these critical challenges. Immune response directed to spike protein of coronaviruses such as SARS-CoV and SARS-CoV-2 and MERS-CoV demonstrated strong neutralizing activity against to these viruses. Indeed, current DNA, mRNA, adenovirus or modified vaccinia Ankara (MVA)-based SARS-CoV-2 vaccination approaches are delivered encouraging results in preclinical and clinical trials. Existing and emerging evidence suggest that durability of immune responses and protective efficacies against new viral variants and to other beta-CoVs of these vaccination approaches remained compromised. Thus, there is an urgent need for development of vaccines that elicit broadly cross-reactive T cells and neutralizing antibody response against new SARS-CoV-2 variants and to other betacoronavirus (beta-CoV) to control current pandemic and future emergence of novel coronaviruses.
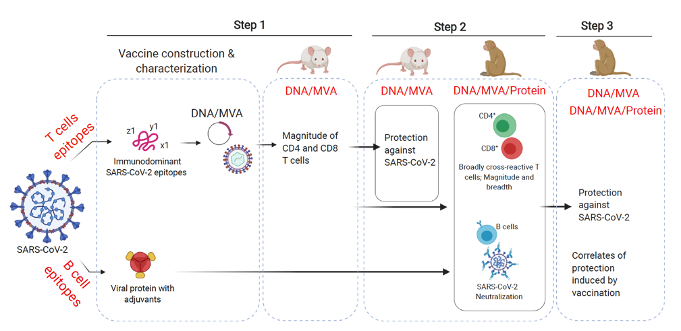
Our nucleic acid (DNA)/Modified Vaccinia virus Ankara (MVA)-based vaccination platform known to rapidly generate broadly cross-reactive T cells in systemic and mucosal compartments and promises to provide long-term protection from SARS-CoV-2, variants and other beta-CoV infections. Our designs leverage the multiepitope approaches derived conserved beta-CoV immunodominant B cell and T cell epitopes that induces broad beta-CoV-specific neutralizing antibodies and T cell breadth in mice and rhesus macaques. Also, we will merge adjuvated SARS-CoV-2 protein typically to generate desirable arm of humoral responses. This DNA/MVA-based multiepitope universal beta-CoV vaccine will be tested in mice and non-human primates for its ability to induce broad beta-CoV-specific neutralizing antibodies and T cell breadth to control replication of SARS-CoV-2, variants and other betacoronavirus (beta-CoV). We use these strategies to define immunological thresholds that required for vaccine efficacy and correlates of protection against COVID-19 infection.
Figure – The schematic of multiepitope, nucleic acid (DNA)/Modified Vaccinia virus Ankara (MVA)/protein-based vaccination approaches SARS-CoV-2, emerging variants and other beta-CoVs.
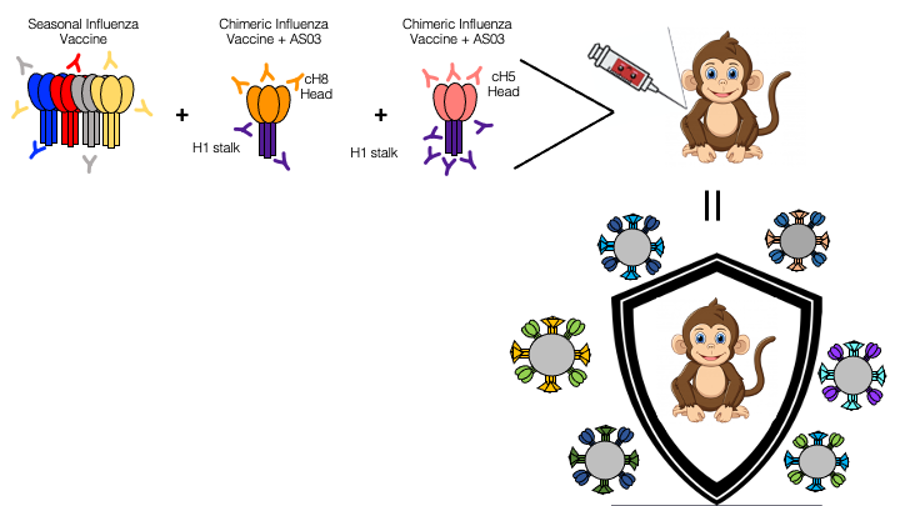
Understanding the immune responses to the seasonal influenza vaccines
To develop more effective influenza vaccines that can protect against variants, it important to characterize the immune response to a primary seasonal influenza vaccine in the rhesus macaque model. Macaques are not traditionally used to model the immune response to influenza, though macaques are more representative of human immune response relative to other animal models. This project also aims to examine different combinations of influenza antigens and immunological adjuvants in the monkey model to determine which merger drives the establishment of long--lived antibody and T cell responses capable of recognizing and eliminating a broad variety of influenza virus strains. We aim to test chimeric HA antigens, delivered either as protein plus adjuvant or mRNA, viral vectors expressing influenza antigens, among other vaccines in the macaque model. Macaques are not traditionally used to model the immune response to influenza, though macaques are more representative of human immune responses to numerous pathogenic species. This provides an opportunity to characterize immune responses to a primary seasonal influenza vaccine in the rhesus macaque model and strengthen this animal model as a viable option for influenza research.
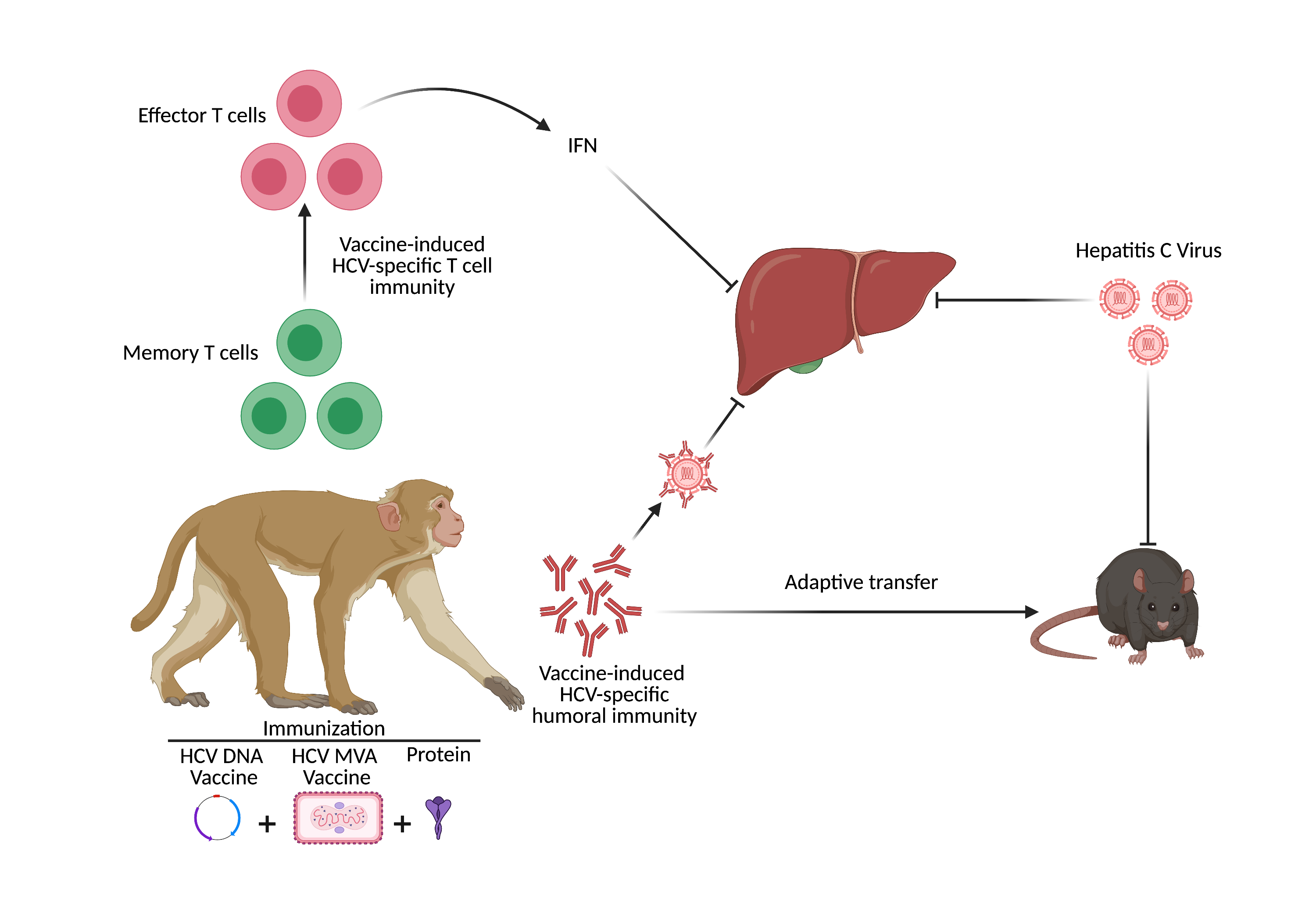
Development of a prophylactic vaccine against Hepatitis C Virus
Our DNA/MVA vaccine platform has in the past elicited strong antibody and T-cell immune responses against HIV. So, here we hypothesize that induction of a strong, broad, and persistent T cell response against HCV antigens in the liver combined with induction of strong neutralizing antibody response will provide protection from HCV infection. Also, different combinations of DNA/MVA/protein/adjuvants vaccination can be harnessed to achieve the desired protective immunity. We are evaluating a series of vaccination regimen to down select an optimal combination that can induce strong and broad T cell immunity and neutralizing antibody responses. We are studying ways to augment the immune responses using molecular adjuvants such as CD40L that will be co-expressed on the HCV-virus like particles (HCV-VLPs). We would also study combinations of vaccine strategies including sequential prime/boost with immunogens from different HCV genotypes with specific antigenic protein boosts and adjuvants to further improve the immune responses.